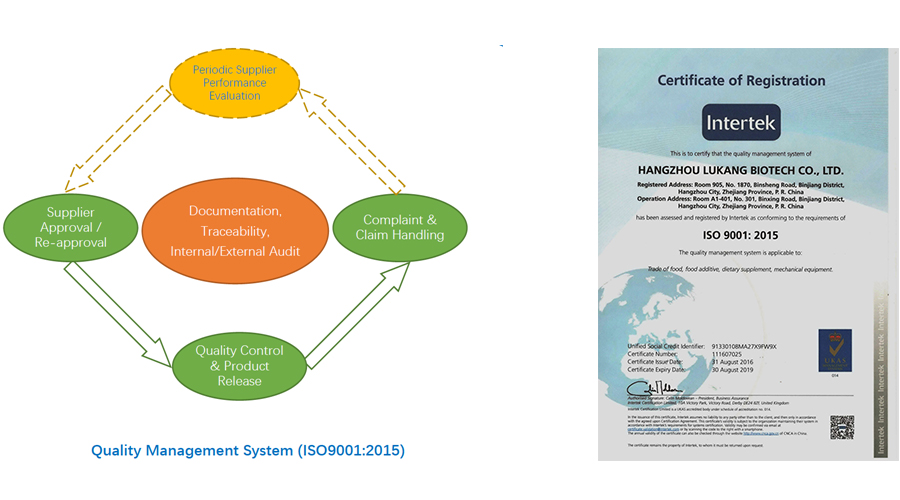
LK-Biotech has established a comprehensive quality management system based on ISO9001:2015 standard. We strictly follow established quality assurance procedures so as to provide products of reliable quality and excellent services to our customers.
We have established a quality manual covering all quality related activities, and put special efforts into the following aspects:
Supplier Approval We purchase only from approved suppliers and have established a procedure for approving/reapproving suppliers, including legal licenses/ certificates confirmation, questionnaire investigation, on-site inspection, periodic performance evaluation, etc. LK-Biotech has professional supplier management staff to supervise suppliers and help them take necessary CAPAs (corrective actions and preventive actions) to eliminate potential risks.
Quality Control & Product Release Our quality staff set up specifications for the products based on the quality requirements from our customers. Sample and related quality documentation (e.g., CoAs, batch records if applicable) of every batch of product must be checked against its specification, and approved by quality staff before the batch is released for delivery.
Complaint & Claim Handling We document every complaint and claim, and take them very seriously. We analyze and determine the root causes, take necessary CAPAs to solve the problems and prevent their reoccurrence.
Traceability Through our documentation system, the whole history of very batch of product can be tracked, including supplier information, retention samples, delivery information, etc.
The effective running of our quality management system is guaranteed by both internal / external audits according to ISO9001:2015 standard.